Predictive Finite Element Modeling
In our effort to understand soft tissue disease and develop new diagnostic and therapeutic technologies, we also make use of the finite element method. Our experience and interest cover all aspects of finite element modeling including constitutive modeling, method development, and application. This work naturally overlaps with many of our other research thrusts such as mechanical testing, imaging, and device evaluation. Specific current projects are finite element models of the right ventricle in which we combine in-vivo imaging in sheep, with DT-MRI, and simple shear testing-derived mechanical properties to quantify ventricular wall stress in health and disease. Similarly, we are combining kinematic and hemodynamic measurements in beating human hearts with mechanical testing data to build high-fidelity, subject-specific finite element models of the human tricuspid valve, see below.
Validation of a subject-specific finite element model of the human tricuspid valve against 2D echo data
Mathur M, Meador WD, Malinowski M, Jazwiec T, Timek TA, Rausch MK. Texas TriValve 1.0: A reverse-engineered, open model of the human tricuspid valve. Engineering with Computers, 2022.
Sree VD, Rausch MK, Tepole AB. Linking microvascular collapse to tissue hypoxia in a multiscale model of pressure ulcer initiation. Biomechanics and Modeling in Mechanobiology, 2019.
Sree VD, Rausch MK, Tepole AB. Towards understanding pressure ulcer formation: coupling an inflammatory regulator network to a tissue scale finite element model, 2019.
van Kelle MAJ, Rausch MK, Kuhl E, Loerakker S. A computational model to predict cell traction-mediated prestretch in the mitral valve. Computer Methods in Biomechanics and Biomedical Engineering, 2019.
Elenes EY, Rausch MK, Rylander CG. Parameteric study of the design variables of an arborizing catheter on dispersal volume using a biphasic computational model. Journal of Medical Diagnostics, 2019.
Rausch MK, Humphrey JD. A computational model of the biochemomechanics of evolving occlusive thrombus. Journal of Elasticity, 2017.
… and more, see Publications.
Thrombus and Blood Clot Mechanics
Thrombotic conditions are the number of cause of death. In fact, 1 in 4 deaths are due to thrombus or blood clot forming in our veins and/or arteries and occluding blood supply to vital organs such as the brain, the lungs, or the heart. During this current COVID-19 pandemic, these numbers of death due to thrombotic conditions are likely to increase as 31% of COVID-19 ICU patients suffer from thromboembolic conditions. We are interested in characterizing the mechanics of blood clot toward a better understanding of the material and why blood clots break off in some patients but not in others.
Test geometry and test modes to determine the nonlinear, dissipative mechanisms of blood clot toward understanding its pre-fracture and fracture behavior.
Sugerman GP, Kakaletsis S, Thakkar P, Chockshi A, Parekh SH, Rausch MK. A whole blood clot thrombus mimic: Constitutive behavior under simple shear.
Wang Y, Kumar S, Nisar A, Bonn M, Rausch MK, Parekh SH. Effect of shear and tensile loading on fibrin molecular structure revealed by coherent Raman spectroscopy.
Kumar S, Wang Y, Rausch MK, Parekh SH. Structural control of fibrin bioactivity by mechanical deformation.
Sugerman GP, Parekh SH, Rausch MK. Nonlinear, dissipative phenomena of whole blood clot mechanics. Soft Matter, 2020. (PDF)
Rausch MK, Humphrey JD. A computational model of the biochemomechanics of evolving occlusive thrombus. Journal of Elasticity, 2017. (PDF)
Y-U Lee, AY Lee, Humphrey JD, Rausch MK. Histological and biomechanical changes in a mouse model of venous thrombus remodeling. Biorheology, 2015. (PDF)
Design & Evaluation of Implantable Cardiovascular Devices
Tricuspid and mitral valve leakage or regurgitation affect millions of Americans. The success of surgical repair is suboptimal with many patients experiencing recurrent regurgitation. Our research addresses significant challenges in the treatment of mitral and tricuspid valve regurgitation. To this end, we test surgical devices and techniques in large animals by combining in-vivo imaging with modeling tools. Additionally, we evaluate commercial devices by combine reverse engineering technology (3D scanning) with mechanical testing tools.
Mathur M, Meador WD, Jazwiec T, Malinowski M, Timek TA, Rausch MK. Tricuspid Valve Annuloplasty Alters Leaflet Mechanics. Annals of Biomedical Engineering, 2020. (PDF)
Mathur M, Meador WD, Jazwiec T, Malinowski M, Timek TA, Rausch MK. The effect of downsizing on the normal tricuspid annulus. Annals of Biomedical Engineering, 2020. (PDF)
Mathur M, Malinowski M, Timek TA, Rausch MK. Tricuspid annuloplasty rings: A quantitative comparison of size, non-planar shape, and stiffness. The Annals of Thoracic Surgery, 2020. (PDF)
Jazwiec T, Malinowski M, Ferguson H, Wodarek J, Quay N, Bush J, Goehler M, Parker J, Rausch MK, Timek TA. Effect of variable annular reduction on functional tricuspid regurgitation and right ventricular dynamics in an ovine model of tachycardia induced cardiomyopathy. The Journal of Thoracic and Cardiovascular Surgery, 2019. (in print: PDF)
Malinowski M, Jazwiec T, Quay N, Goehler M, Rausch MK, Timek TA. The influence of tricuspid annuloplasty prostheses on ovine annular geometry and kinematics. The Journal of Thoracic and Cardiovascular Surgery, 2019. (in print: PDF)
Malinowski M, Jazwiec T, Goehler M, Bush J, Quay N, Ferguson H, Rausch MK, Timek TA. Impact of tricuspid annular size reduction on right ventricular function, geometry and strain. European Journal of Cardio-thoracic Surgery, 2019. (PDF)
… and more, see Publications.
Tricuspid and Right Ventricular In-vivo Function
The right ventricle and the tricuspid valve, for a long time, were considered unimportant in comparison to the left ventricle and the mitral valve. However, it has become quite clear that a healthy right ventricle and tricuspid valve are vital to our well-being. Because the right and left ventricles are different, despite their anatomic proximity and connectedness, we cannot simply extrapolate what we know about the one onto the other. Thus, there is a significant gap in our knowledge which we are aiming to fill. Similarly, the tricuspid valve varies significantly from the mitral valve. Here, too, much work is needed to improve current surgical outcomes and medical treatment.
In-vivo strains of the tricuspid valve leaflets from sonomicrometry and a subdivision surface-based kinematics
Meador WD, Mathur M, Rausch MK. Tricuspid Valve Biomechanics: A Brief Review. In: Advances in Heart Valve Biomechanics, Springer, 2019. (PDF)
Mathur M, Jazwiec T, Meador WD, Malinowski M, Goehler M, Ferguson H, Timek TA, Rausch MK. Tricuspid valve leaflet strains in the beating ovine heart. Biomechanics and Modeling in Mechanobiology, 2019. (PDF)
Rausch MK, Mathur M, Meador WD. Biomechanics of the Tricuspid Annulus: A Review of the Annulus’ In-Vivo Dynamics With Emphasis on Ovine Data. Journal of Applied Mathematics and Mechanics, 2019 (PDF).
Malinowski M, Jazwiec T, Goehler M, Quay N, Bush J, Jovinge S, Rausch MK, Timek TA. Sonomicrometry Derived Three-Dimensional Geometry of the Human Tricuspid Annulus. Journal of Thoracic and Cardiovascular Surgery, 2019. (PDF)
Meador WD, Malinowski M, Jazwiec T, Goehler M, Quay N, Timek TA, Rausch MK. A fiduciary marker-based framework to assess heterogeneity and anisotropy of right ventricular epicardial strains in the beating ovine heart. Journal of Biomechanics, 2018. (PDF)
Rausch MK, Malinowski M, Wilton P, Khaghani A, Timek TA. Engineering Analysis of Tricuspid Annular Dynamics in the Beating Ovine Heart. Annals of Biomedical Engineering, 2017. (PDF)
… and more, see Publications.
Histomechanical Characterization and Constitutive Modeling of Biological Soft Tissues
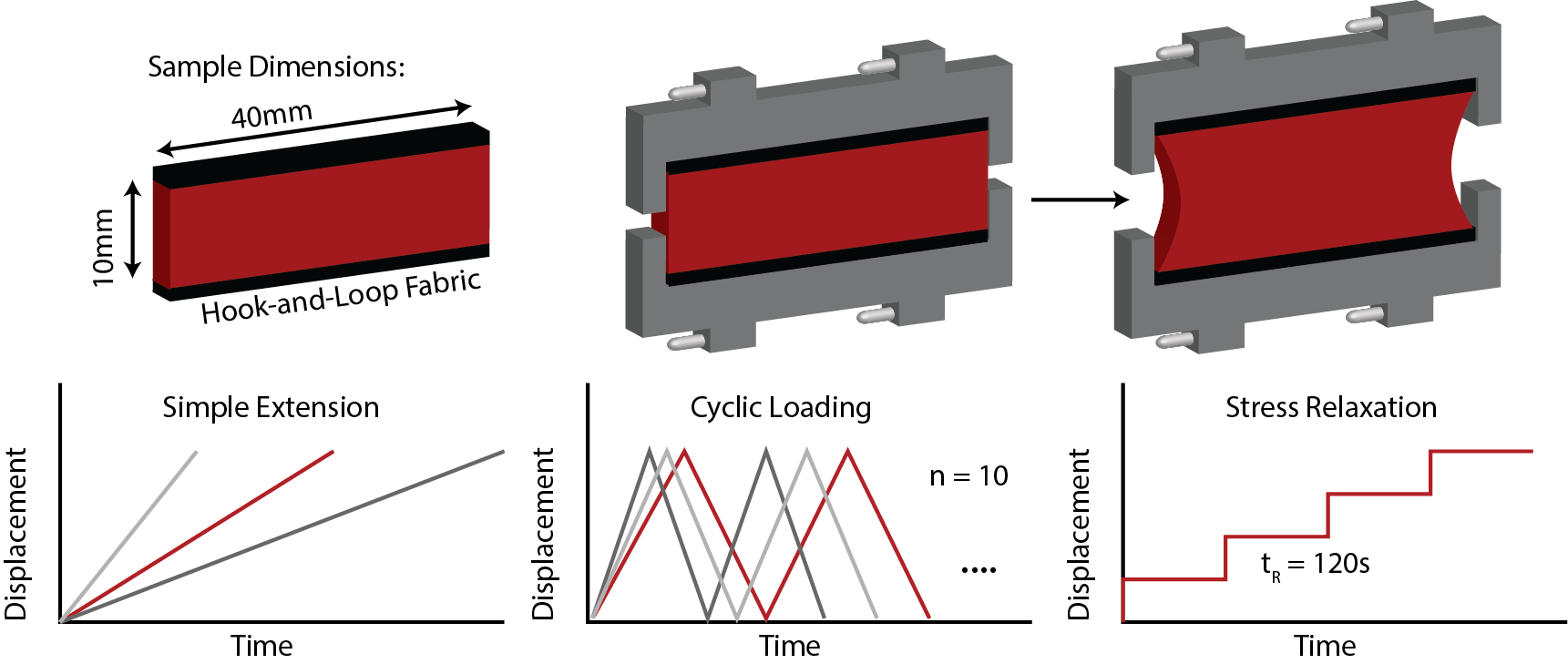
We use planar biaxial testing, uniaxial testing, bulge testing, digital image correlation, optical coherence tomography, and 2-photon/fluorescent microscopy to characterize the spatiotemporal histomechanical propeties of biological soft tissues. Current projects look at changes in murine skin during the process of aging and the formation of pressure ulcers, healthy and diseased human heart valve tissue. Additionally, we are interested in human fetal membranes and microstructural/mechanical changes that may contribute to preterm premature rupture of these membranes.
Histology-derived compositional and structural characterization of young and old mouse skin
Smith KJ, Mathur M, Meador WD, Philips-Garcia B, Sugerman GP, Menta AK, Jazwiec T, Malinowski M, TImek TA, Rausch MK. Tricuspid Chordae Tendineae Mechanics: Insertion Site, Leaflet, and Size-Specific Analysis and Constitutive Modelling. Journal of Experimental Mechanics, 2020. (PDF)
Meador WD, Mathur M, Jazwiec T, Malinowksi M, Bersi MR, Timek TA, Rausch MK. A detailed mechanical and microstructural analysis of the ovine tricuspid valve leaflets. Acta Biomaterialia, 2020. (PDF)
Meador WD, Sugerman GP, Story HM, Seifert AW, Bersi MR, Tepole AB, Rausch MK. The regional-dependent biaxial behavior of young and aged mouse skin: A detailed histomechanical characterization, residual strain analysis, and constitutive model. Acta Biomaterialia, 2020. (PDF)
Histological analysis of ovine right ventricular myocardium samples. We quantified myofiber orientation in ten slices across the transmural direction and the longitudinal direction and found that myocardial microstructural organization is more complex than previously appreciated